What Is the Best Buffer Chemistry
Determine whether each addition. A 5000 mL buffer solution is 0100 M in HNO2 and 0150 M in KNO2.

What Is A Buffer Solution Chemistry Chemtalk
A buffer solution resists a change in pH when H or OH- ions are added.

. When H or OH- ions are added to a solution from a strong acid or base the weak acid or base is the best source of other H or OH- ions. Which buffer system is the best choice to create a buffer with pH 720. A HC2H3O2KC2H3O2 b HClO2KClO2 c NH3NH4Cl d HClOKClO.
The ions form to make water H2O. A buffer solution will prolong a reaction if added. The remaining conjugate reacts with more H or OH- to make a compound.
For the best system calculate the ratio of the masses of the buffer components required to make the buffer.

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
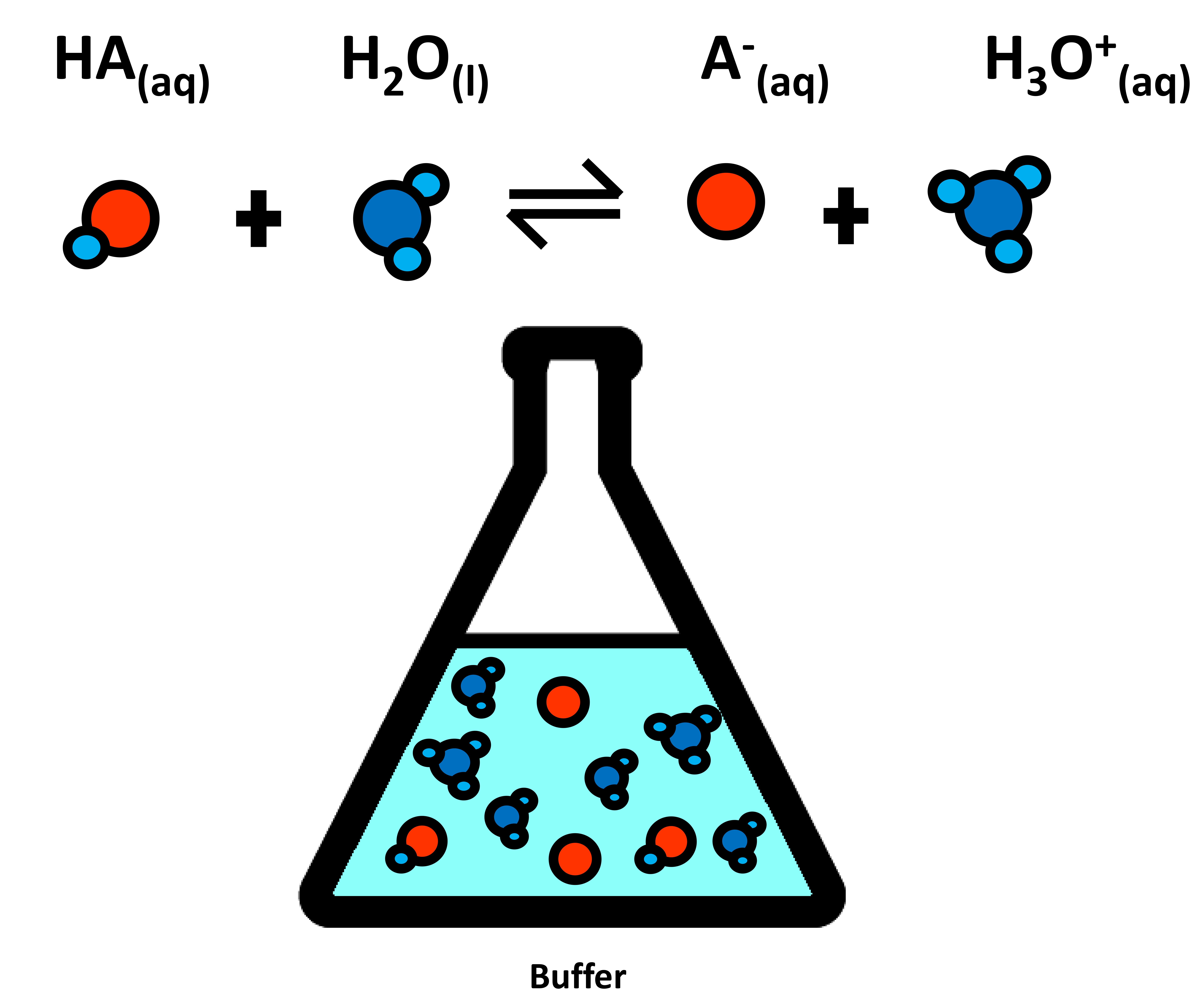
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
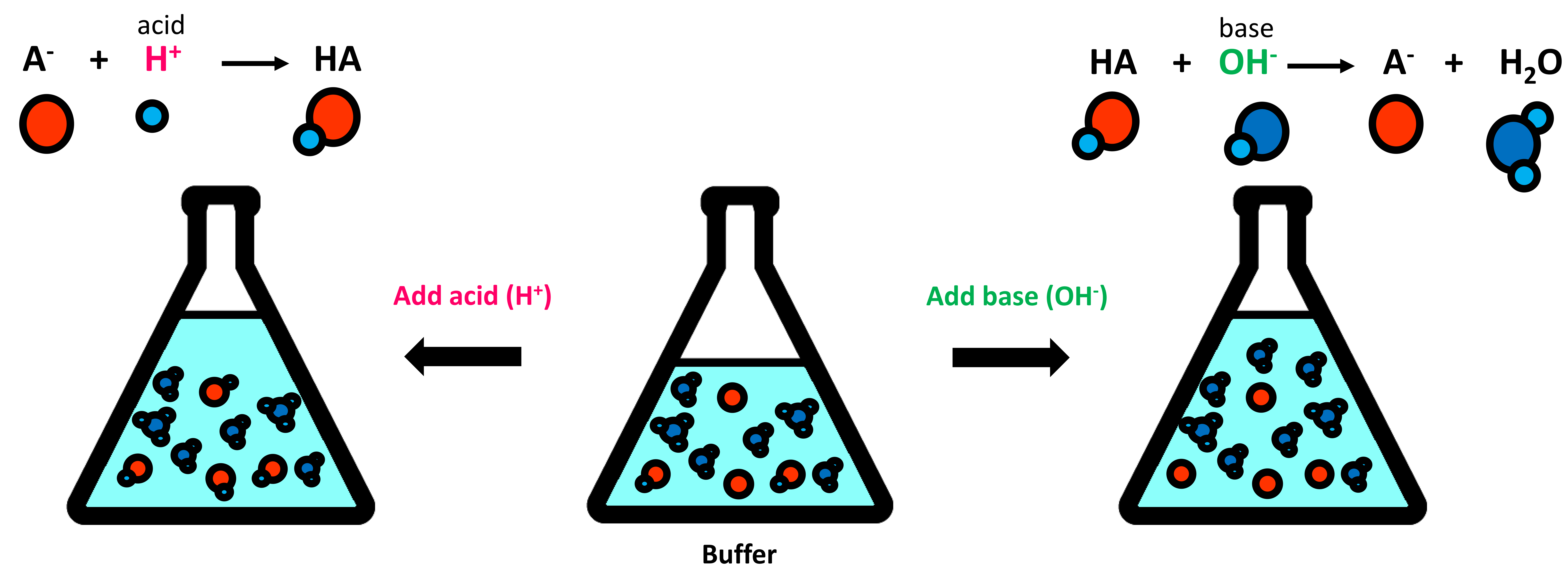
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
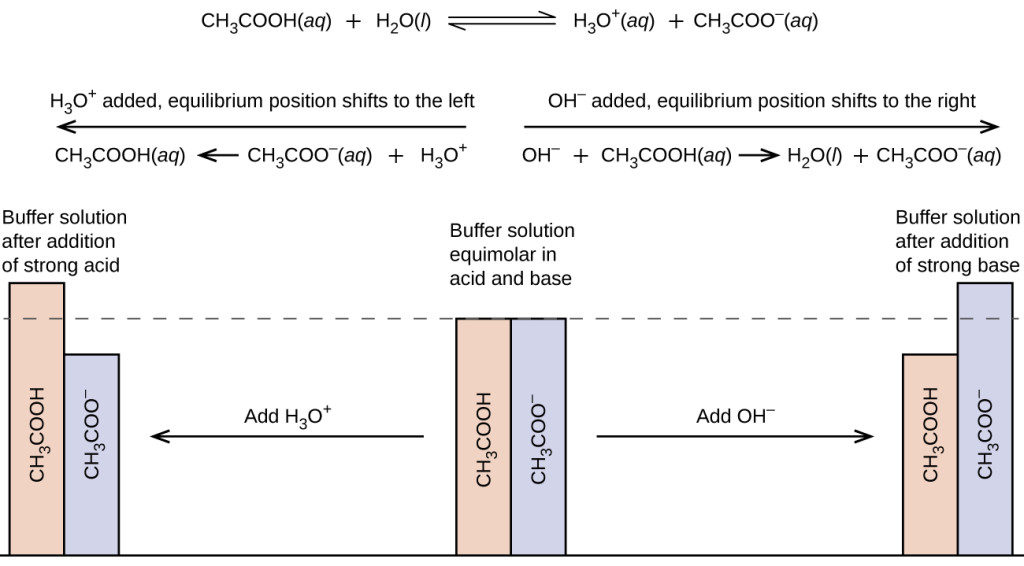
No comments for "What Is the Best Buffer Chemistry"
Post a Comment